1. Explain / Give the mechanism for Claisen Or Claisen-Schmidt reaction.
Claisen or Claisen-Schmidt reaction: Benzaldehyde reacts with aliphatic aldehydes or ketones in presence of NaOH forming a, β - unsaturated aldehyde or ketone.
NaOH
C6H5 – CHO + CH3 – CHO → C6H5 – CH = CH – CHO + H2O
Similarly, NaOH
C6H5 – CHO + CH3 – CO – CH3 → C6H5 – CH = CH – CO – CH3 + H2O
Mechanism:
The carbanion formed from acetaldehyde or acetone brings forth a nucleophilic attack on the carbonyl carbon of Benzaldehyde. The aldol type of product undergoes β-elimination (of water).
Step 1: The carbanion is formed as the a-hydrogen atom is removed as a proton by the base.
Step 2: The carbanion (Nucleophile) attacks the carbonyl carbon of Benzaldehyde.
Step 3: β-elimination of water.
2. Explain / Write the mechanism of crossed aldol condensation (of acetone).
Crossed aldol condensation: When an aldehyde and a ketone with a-hydrogen atom react in presence of NaOH forming Aldol.
CH3
|
CH3 – CO – CH3 + CH3 – CHO → CH3 – C – CH2 – CHO
|
OH
Mechanism:
Step 1: The carbanion is formed as the a-hydrogen atom is removed as a proton by the base.
Step 2: The carbanion attacks the carbonyl carbon of unionised ketone molecule.
Step 3: The alkoxide ion formed is protonated by water to give ‘Aldol’.
3. Explain the mechanism of aldol condensation in acetone.
Aldol condensation: When ketones with a-hydrogen atom react in presence of NaOH forming Ketol.
CH3 O
| ||
CH3 – CO – CH3 + CH3 – CO – CH3 → CH3 – C – CH2 – C – CH3
|
OH
Mechanism:
Step 1: The carbanion is formed as the a-hydrogen atom is removed as a proton by the base.
Step 2: The carbanion attacks the carbonyl carbon of another unionised ketone molecule.
Step 3: The alkoxide ion formed is protonated by water to give ‘Ketol’.
4. Explain the mechanism of aldol condensation of acetaldehyde. Or Discuss the mechanism involved in aldol condensation of acetaldehyde.
Aldol condensation: When aldehydes with a-hydrogen atom react in presence of NaOH forming Aldol.
CH3 O
| ||
CH3 – CO – CH3 + CH3 – CHO → CH3 – C – CH2 – C – H
|
OH
Mechanism:
Aldol condensation is catalysed by base. The carbanion generated is nucleophilic in nature. Hence it can bring about nucleophilic attack on carbonyl group.
Step 1: The carbanion is formed as the a-hydrogen atom is removed as a proton by the base.
Step 2: The carbanion attacks the carbonyl carbon of another unionised acetaldehyde molecule.
Step 3: The alkoxide ion formed is protonated by water to give ‘Aldol’.
5. Discuss the mechanism of aldol condensation.
Answer: Q.No. 2 or 3 or 4
6. Explain the mechanism of Cannizzaro reaction.
Cannizzaro reaction: Aldehydes without a-hydrogen atom when heated with concentrated NaOH involves self oxidation of one aldehyde molecule to carboxylic acid and self reduction of the other aldehyde molecule to a primary alcohol.
NaOH
C6H5 – CHO + C6H5 – CHO → C6H5 – COONa + C6H5 – CH2 – OH
The mechanism involves the transfer of hydride ion from one molecule of benzaldehyde to the other molecule.
Step 1: Nucleophilic attack by OH – ion on carbonyl carbon
Step 2: Transfer of hydride ion from the anion to carbonyl carbon of another molecule.
Step 3: The benzyloxide ion picks up the acidic proton from benzoic acid to give benzyl alcohol.
7. Explain 'Popott's rule' with an example.
During oxidation of unsymmetric ketones with oxidising agent which brings about the cleavage of C–C bond, the smaller alkyl group goes preferentially with the carbonyl group resulting in the carboxylic acids.
8. How is acetone converted to - i) mesityl oxide ii) mesitylene?
i) Acetone reacts with dry hydrogen chloride forms mesityl oxide.
Dry. HCl
CH3 – CO – CH3+ H2CH – CO – CH3 → (H3C)2 – C = CH – CO – CH3
–H2O
Mesityl oxide
4-methyl pent-3-ene-2-one
ii) In presence of Conc. H2SO4 three molecules of acetone condense to give mesitylene (1, 3, 5 trimethyl benzene)
9. Write notes on i) Perkin’s reaction and ii) Knoevenagal reaction iii) Stephen’s reaction.
i) Perkin’s reaction: When benzaldehyde is heated with sodium salt of acetic acid in presence of acetic anhydride, it forms Cinnamic acid.
CH3COONa
C6H5–CH = O + CH3–CO– O –CO–CH3 → C6H5–CH = CH–COOH + CH3–COOH
Acetic anhydride Δ Cinnamic acid
Mechanism:
Sodium acetate is the base that generates a carbanion at the a-carbon in the acetic anhydride. This brings forth nucleophilic attack on the carbonyl carbon forming β-hydroxy acid, water gets removed from this by β-elimination.
ii) Knoevenagal reaction: Benzaldehyde condenses with malonic acid in presence of pyridine forming cinnamic acid, pyridine is the basic catalyst here.
Pyridine CO2
C6H5 – CH = O + H2C(COOH)2 → C6H5 – CH = C(COOH)2 → C6H5 – CH = CH – COOH
Malonic acid Δ Cinnamic acid
iii) Stephen’s reaction: Aldehyde can be prepared by the reduction of alkyl cyanide dissolved in ether with Stannous chloride and hydrochloric acid.
H – H SnCl2
CH3 – C ≡ N → CH3CH = NH.HCl
Methyl cyanide HCl Iminimum hydro chloride
O – H2 Hydrolysis
CH3 CH = NH.HCl → CH3CHO + NH4Cl
Acetaldehyde
10. Give the
following reactions (i) Benzoin
Condensation (ii) Knoevenagal
reaction
(i) Benzoin Condensation:
(i) Benzoin Condensation:
When
benzaldehyde is refluxed with aqueous alcoholic potassium cyanide – α - hydroxy
ketone called benzoin is formed. Cyanide ion (CN–) is the specific
catalyst in this reaction.
Benzoin can be
considered as dimer of benzaldehyde.
O O
|| alc.
KCN ||
C6H5CH
= O + H – C – C6H5 → C6H5CHOH – C
– C6H5
Benzoin
(ii)
Knoevenagal reaction:
Benzaldehyde
condenses with malonic acid in presence of pyridine forming cinnamic acid;
pyridine is the basic catalyst here.
Pyridine CO2
C6H5CH
= O + H2C(COOH)2 → C6H5CH
= C(COOH)2 → C6H5CH
= CHCOOH
Malonic acid Δ Cinnamic acid
11. Write
the differences between Acetaldehyde and Benzaldehyde Or
Compare
Aliphatic aldehyde and Aromatic aldehyde
Reactions
|
Acetaldehyde
CH3CHO |
Benzaldehyde
C6H5CHO |
Aliphatic aldehyde
|
Aromatic aldehyde
|
|
1. With Fehling’s solution
|
Gives a Red precipitate.
|
No reaction
|
2. With Ammonia
|
Forms Simple
Addition product |
Forms Complex
Condensation product |
3. With Caustic soda
|
Undergoes Aldol condensation
|
Undergoes Cannizzaro reaction
|
4. With Primary amines
|
Does not form Schiff’s base
|
Forms Schiff’s base.
|
5. With Chlorine
6. Polymerisation 7. Electrophilic substitution 8. With Schiff’s reagent |
Does not form Acetyl chloride
Undergoes Polymerisation Does not undergo Gives Pink colour in cold |
Forms Benzoyl chloride
Does not polymerise Undergoes at the meta position Gives Pink colour |
12. Write
a note on i) Clemmenson reduction and
ii) Knoevenagel reaction
i) Clemmenson reduction : Aldehydes and ketones
can be reduced to Hydrocarbons by Zinc amalgam and Con. HCl.This reaction proceeds by electron addition to carbonyl carbon followed by protonation. Zinc metal is the electron source
In the absence of mercury, hydrogen gas will be evolved and the reduction
is incomplete. This reduction is called Clemmenson reduction.
Clemmenson reduction
|
HCHO
|
CH3CHO
|
C6H5CHO
|
CH3COCH3
|
C6H5COCH3
|
C6H5COC6H5
|
Zn / Hg +
Conc. Hcl
|
←--------- > C = O Group reduced to – CH2 – Group to give
Hydrocarbons ---------→
CH4 CH3CH3 C6
H5CH3 CH3CH2CH3 C6H5CH2CH3 C6H5CH2C6H5
|
|||||
ii) Knoevenagel reaction : Benzaldehyde
condenses with malonic acid in presence of pyridine forming cinnamic acid;
pyridine is the basic catalyst here.
Pyridine CO2
C6H5CH
= O + H2C(COOH)2 → C6H5CH
= C(COOH)2 → C6H5CH
= CHCOOH
Malonic acid Δ Cinnamic acid
13. Illustrate
the reducing property of acetaldehyde with examples
Because
aldehydes are easily oxidised, they are reducing agents. The reduce Ammonical
silver nitrate (Tollen’s reagent) to metallic Silver and Fehling’s solution (Copper
sulphate, Sodium potassium tartrate) to red Cuprous oxide.
CH3CHO + 2Ag+ + 3OH–
→ CH3COO– + 2Ag + 2H2O
Acetaldehyde Acetate ion (Silver mirror)
CH3CHO + 2Cu2+ + 5OH–
→ CH3COO– + Cu2O + 3H2O
(Blue) (Red
precipitate)
Cupric ion Cuprous ion
14. Write the differences between acetaldehyde and acetone.
S.No
|
Reactions
|
Acetaldehyde
CH3CHO
|
Acetone
CH3COCH3
|
1
|
With Fehling’s solution.
|
Gives a red precipitate
|
Does not react.
|
2
|
With Tollen’s reagent.
|
Gives silver mirror.
|
No silver mirror.
|
3
|
Oxidation
|
Gives acetic acid
|
Gives acetic acid
with loss of one
Carbon atom.
|
4
|
Reduction with NaBH4
|
Gives ethanol
(primary alcohol)
|
Gives isopropyl alcohol (secondary alcohol)
|
5
|
With NH3
|
Simple addition product is formed.
|
Forms complex ketonic amine.
|
6
|
Iodoform reaction
|
Forms iodoform and formic acid.
|
Forms iodoform and acetic acid.
|
7
|
Polymerisation
|
Forms paraldehyde
|
Forms condensation products.
|
8
|
With Schiff’s reagent.
|
Pink colour appears in cold.
|
No pink colour in cold.
|
9
|
Warming with NaOH
|
A brown resinous mass.
|
No resinous mass.
|
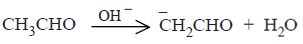

















very nice thank u
ReplyDeleteThank you for your appreciation.
DeleteThank You Sir
ReplyDelete